Details of the Drug
General Information of Drug (ID: DMR28HG)
| Drug Name |
5'-Deoxy-5'-(Methylthio)-Tubercidin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
5'-methylthiotubercidin; 5'-DEOXY-5'-(METHYLTHIO)-TUBERCIDIN; 2-(4-AMINO-PYRROLO[2,3-D]PYRIMIDIN-7-YL)-5-METHYLSULFANYLMETHYL-TETRAHYDRO-FURAN-3,4-DIOL; MTH; 2qtg; 7-(5-S-methyl-5-thio-beta-D-ribofuranosyl)-7H-pyrrolo(2,3-d)pyrimidin-4-amine; 7-(5-S-methyl-5-thio-beta-D-ribofuranosyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; 61893-98-9; 1nc1; AC1Q7DT3; AC1L4MN1; 5'-(methylsulfanyl)tubercidin; CHEMBL551561; DB02933; 7-(5-s-methyl-5-thio-; A-d-ribofuranosyl)-7h-pyrrolo[2,3-d]pyrimidin-4-amine; 7H-Pyrrolo(2,3-d)pyrimidin-4-amine,
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
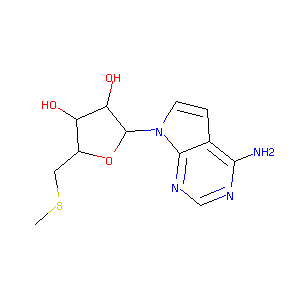 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 296.35 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
