Details of the Drug
General Information of Drug (ID: DMR2MUG)
| Drug Name |
ACULEACIN A
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aculeacin A; 58814-86-1; AC1Q6G0M; AC1L3U84; YKPHLXGEPNYRPY-UHFFFAOYSA-N; LS-14909; n-{23-[1,2-dihydroxy-2-(4-hydroxyphenyl)ethyl]-2,11,12,15-tetrahydroxy-6,20-bis(1-hydroxyethyl)-16-methyl-5,8,14,19,22,25-hexaoxotetracosahydro-1h-dipyrrolo[2,1-c:2',1'-l][1,4,7,10,13,16]hexaazacyclohenicosin-9-yl}hexadecanamide; Echinocandin B, 1-((4R,5R)-4,5-dihydroxy-N(sup 2)-(1-oxohexadecyl)-L-ornithine)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
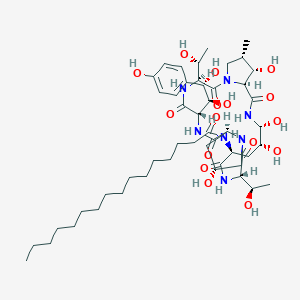 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1036.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 20 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 14 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 16 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
