Details of the Drug
General Information of Drug (ID: DMR7U8F)
| Drug Name |
Etoperidone
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Etoperidona; Etoperidona [INN-Spanish]; Etoperidona [Spanish]; Etoperidone [INN]; Etoperidonum; Etoperidonum [INN-Latin]; ETOPERIDONE; IZBNNCFOBMGTQX-UHFFFAOYSA-N; KAI6MVO39Z; L001188; ZINC3830815; 2-[3-[4-(3-chlorophenyl)piperazin-1-yl]propyl]-4,5-diethyl-1,2,4-triazol-3-one; 52942-31-1; AC1L242J; AC1Q3SR1; ACM52942311; BDBM82438; CAS_52942-31-1; CHEBI:135589; CHEMBL1743259; DB09194; DTXSID0023034; NSC_40589; PDSP1_000523; PDSP2_000521; SCHEMBL49314; UNII-KAI6MVO39Z
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Structure |
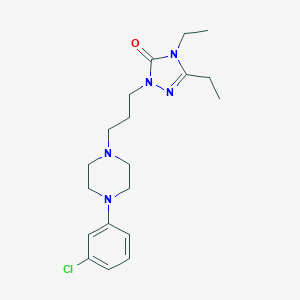 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 377.9 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | |||||
| Rotatable Bond Count (rotbonds) | 7 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
