Details of the Drug
General Information of Drug (ID: DMRD8HK)
| Drug Name |
TAK-683
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(5S)-6-[6-[(1E,3R,5Z)-3-Hydroxyundeca-1,5-dienyl]pyridin-2-yl]hexane-1,5-diol; U 75302; U-75302; 119477-85-9; (5S)-6-[6-[(1E,3R,5Z)-3-hydroxyundeca-1,5-dienyl]pyridin-2-yl]hexane-1,5-diol; 6-(6-(3-Hydroxy-1,5-undecadien-1-yl)-2-pyridinyl)-1,5-hexanediol; U75302; AC1O6A9C; SCHEMBL1894359; GTPL3325; ZINC4489632; SR-01000946976; SR-01000946976-1; 1,5-Hexanediol, 6-(6-(3-hydroxy-1,5-undecadienyl)-2-pyridinyl)-,
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
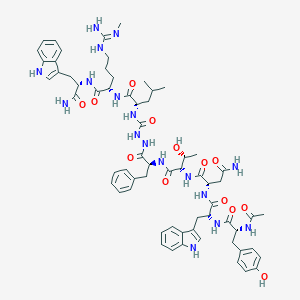 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 1298.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 34 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 18 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 14 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 02 Neoplasm | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 2C82 Prostate cancer | |||||||||||||||||||||||
| The Studied Tissue | Prostate | |||||||||||||||||||||||
| The Studied Disease | Prostate cancer [ICD-11:2C82] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
