Details of the Drug
General Information of Drug (ID: DMRDUC0)
| Drug Name |
Elagolix sodium
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Elagolix sodium salt; UNII-5948VUI423; Elagolix sodium [USAN]; 5948VUI423; Elagolix sodium (USAN); SCHEMBL1641994; NBI 56418NA; NBI-56418-NA; MolPort-003-984-965; NBI-56418 NA; DQYGXRQUFSRDCH-UQIIZPHYSA-M; AKOS030524154; VA12044; KS-0000063K; KB-76766; HY-14369; AC-29671; CS-0003317; D09336
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
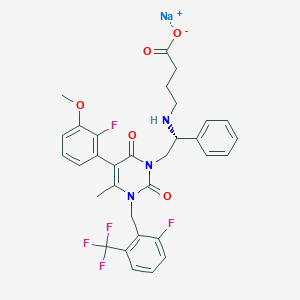 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 |
Molecular Weight | 653.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 12 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
