Details of the Drug
General Information of Drug (ID: DMRZLU4)
| Drug Name |
BAY 86-5044
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Lonaprisan; ZK-230211; 211254-73-8; UNII-F5Z5EL4D26; F5Z5EL4D26; AC1OCFKM; ZK-PRA; Lonaprisan [USAN:INN]; Lonaprisan (USAN/INN); SCHEMBL977182; CHEMBL146032; zk230211; BDBM50409115; BAY86-5044; ZK 230211; ZK 232011; D10016; Z-3103; (8S,11R,13S,14S,17S)-11-(4-ACETYLPHENYL)-17-HYDROXY-13-METHYL-17-(PERFLUOROETHYL)-6,7,8,11,12,13,14,15,16,17-DECAHYDRO-1H-CYCLOPENTA[A]PHENANTHREN-3(2H)-ONE; Lonaprisan
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
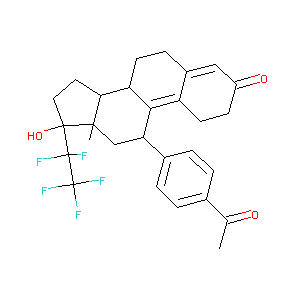 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 508.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
