| Drug Name |
DEOXYADENOSINE
|
| Synonyms |
CHEMBL416340; 9H-Purin-6-amine, 9-(2-deoxy-.beta.-D-ribofuranosyl)-; 9-(2-deoxypentofuranosyl)-9H-purin-6-amine; .beta.-D-erythro-Pentofuranoside, adenine-9 2-deoxy-; .beta.-D-Ribofuranose,2-dideoxy-; 9H-Purin-6-amine, 9-(2-deoxy-.beta.-D-erythro-pentofuranosyl)-; SR-01000397552; 13276-53-4; dAdo
|
| Drug Type |
Small molecular drug
|
| Structure |
|
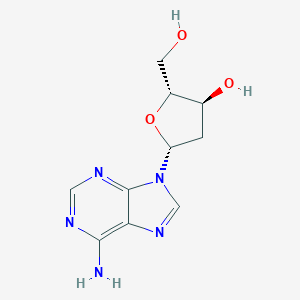 |
|
3D MOL
|
2D MOL
|
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight (mw) |
251.24 |
|
| Logarithm of the Partition Coefficient (xlogp) |
-0.5 |
| Rotatable Bond Count (rotbonds) |
2 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
7 |
| Chemical Identifiers |
- Formula
- C10H13N5O3
- IUPAC Name
(2R,3S,5R)-5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-ol - Canonical SMILES
-
C1[C@@H]([C@H](O[C@H]1N2C=NC3=C(N=CN=C32)N)CO)O
- InChI
-
InChI=1S/C10H13N5O3/c11-9-8-10(13-3-12-9)15(4-14-8)7-1-5(17)6(2-16)18-7/h3-7,16-17H,1-2H2,(H2,11,12,13)/t5-,6+,7+/m0/s1
- InChIKey
-
OLXZPDWKRNYJJZ-RRKCRQDMSA-N
|
| Cross-matching ID |
- PubChem CID
- 13730
- ChEBI ID
-
- CAS Number
-
- TTD ID
- D0E0OJ
|
|
|
|
|
|
|
|



