Details of the Drug
General Information of Drug (ID: DMSDYZQ)
| Drug Name |
2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
gamma-Thujaplicin; 5-Isopropyltropolone; 672-76-4; gamma-Thujaplicine; THUJAPLICIN, ALPHA; .gamma.-Thujaplicin; 2,4,6-Cycloheptatrien-1-one, 2-hydroxy-5-(1-methylethyl)-; NSC43338; .gamma.-Thujaplicine; 2-Hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one; NSC402794; NSC 43338; NSC 18805; NSC 402794; BRN 2044323; AI3-28399; MLS002608534; MLS002702822; CHEMBL1275999; CHEBI:10578; NSC18805; 2,4,6-Cycloheptatrien-1-one, 2-hydroxy-5-isopropyl-; 2-hydroxy-5-isopropyl-cyclohepta-2,4,6-trien-1-one; WLN: L7VJ BQ EY1& SMR001527281
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
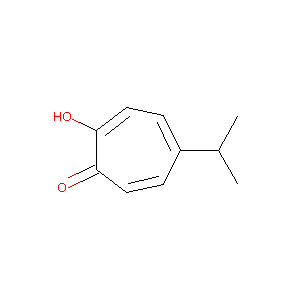 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 164.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
