Details of the Drug
General Information of Drug (ID: DMSQ1JV)
| Drug Name |
LAS-34273
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | Bretaris; Eklira; Aclidinium bromide; LAS-W-330; [1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]oct-8-yl] 2-hydroxy-2,2-dithiophen-2-yl-acetate Bromide | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
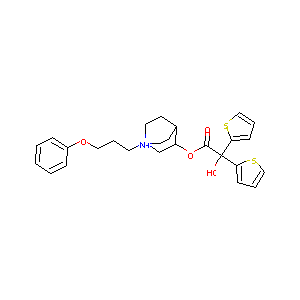 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 |
Molecular Weight | 564.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic obstructive pulmonary disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA22 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
