Details of the Drug
General Information of Drug (ID: DMSUFW6)
| Drug Name |
SSR-125047
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tolrestat; TOLRESTAT; 82964-04-3; Alredase; Lorestat; Tolrestatin; Tolrestatum; Tolrestatum [Latin]; AY-27773; 2-(6-Methoxy-N-methyl-5-(trifluoromethyl)naphthalene-1-carbothioamido)acetic acid; UNII-0T93LG5NMK; AY 27773; AY-27,773; CHEMBL436; 0T93LG5NMK; CHEBI:48549; LUBHDINQXIHVLS-UHFFFAOYSA-N; N-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]carbonothioyl}-N-methylglycine; NCGC00183862-01; N-(6-Methoxythio-5-(trifluoromethyl)-1-naphthoyl)sarcosine; N-{[6-methoxy-5-(trifluoromethyl)-1-naphthyl]carbonothioyl}-N-methylglycine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
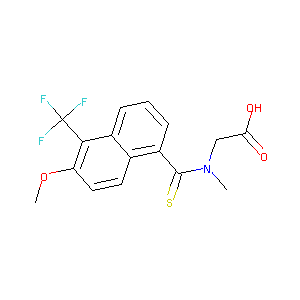 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 357.3 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.7 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Schizophrenia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 6A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
