Details of the Drug
General Information of Drug (ID: DMSWUGE)
| Drug Name |
Telbivudine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Epavudine; LLT; LdT; Sebivo; Telbivudin; Tyzeka; Telbivudine [USAN]; LDT600; NB 02B; NV 02B; L-Deoxythymidine; L-Thymidine; L-dT; LDT-600; NV-02B; Tyzeka (TN); Beta-L-Thymidine; Telbivudine (USAN/INN); Tyzake/Sebivo (TN); Tyzeka, Sebivo, Telbivudine; 1-(2-Deoxy-beta-L-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione; 1-(2-Deoxy-beta-L-erythropentafuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione; 1-[(2S,4R,5S)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione; 2'-Deoxy-L-thymidine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiviral Agents
|
||||||||||||||||||||||
| Affected Organisms |
Hepatitis B virus
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
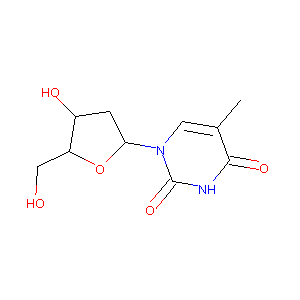 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 242.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Telbivudine (Comorbidity)
|
|||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Nucleoside and nucleotide analogs in the treatment of chronic hepatitis B. Enferm Infecc Microbiol Clin. 2008 May;26 Suppl 7:32-8. | ||||
|---|---|---|---|---|---|
| 2 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Nucleos(t)ide analogues for hepatitis B virus: strategies for long-term success. Best Pract Res Clin Gastroenterol. 2008;22(6):1081-92. | ||||
| 5 | Insights into the mechanisms of telbivudine-induced myopathy associated with mitochondrial dysfunction. Chem Biol Interact. 2023 Sep 25;383:110692. doi: 10.1016/j.cbi.2023.110692. Epub 2023 Sep 1. | ||||
| 6 | Carrion C, Espinosa E, Herrero A, Garcia B "Possible vincristine-isoniazid interaction." Ann Pharmacother 29 (1995): 201. [PMID: 7756727] | ||||
