Details of the Drug
General Information of Drug (ID: DMT45MU)
| Drug Name |
Rosiglitazone XR
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Avandia; Nyracta; Venvia; Rosiglitazone Maleate [USAN]; Rosiglitazone maleate; BRL 49653C; Avandia (TN); Avandiaadministration for 6-12 weeks; BRL 49653-C; BRL-49653C; SB-206846; SB-210232; Rosiglitazone maleate (JAN/USAN); (+-)-5-(p-(2-(Methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione maleate (1:1); (+-)-5-[[4-2-(methyl]-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione,(Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione,5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-,(2Z)-2-butenedioate; 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-{[4-({2-[methyl(pyridin-2-yl)amino]ethyl}oxy)phenyl]methyl}-1,3-thiazolidine-2,4-dione (2Z)-but-2-enedioate
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
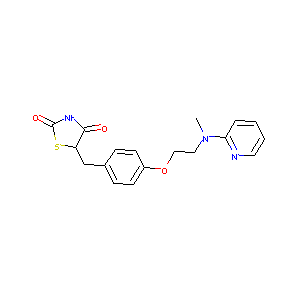 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 473.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 10 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Type-2 diabetes | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A11 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
