Details of the Drug
General Information of Drug (ID: DMTUL9J)
| Drug Name |
Adamantan-1-yl-piperidin-1-yl-methanone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adamantan-1-yl-piperidin-1-yl-methanone; adamantanyl piperidyl ketone; CHEMBL245590; 1-adamantyl(piperidino)methanone; 22508-49-2; (3r,5r,7r)-adamantan-1-yl(piperidin-1-yl)methanone; 1-adamantyl(piperidin-1-yl)methanone; SMR000117086; AC1LBKY9; Maybridge1_008125; Oprea1_633750; Oprea1_290126; MLS000526612; Piperidino(1-adamantyl) ketone; SCHEMBL3457055; SCHEMBL19841773; CTK7F5375; HMS564J07; BURUIZXRHQWZQI-UHFFFAOYSA-N; MolPort-001-930-823; 1-(1-Adamantylcarbonyl)piperidine; HMS2178P18; ZINC4031797
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
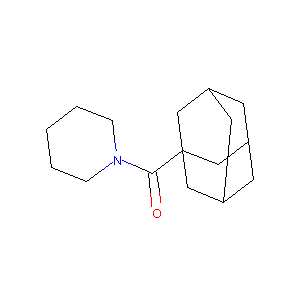 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 247.38 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
