Details of the Drug
General Information of Drug (ID: DMU5NRA)
| Drug Name |
3,4,5-trimethoxy-N-(thiazol-2-yl)benzamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3,4,5-Trimethoxy-N-(1,3-thiazol-2-yl)benzamide; 50591-71-4; AC1LCU6P; Cambridge id 5149479; Oprea1_821264; MLS000027375; CHEMBL225354; ZINC45264; XSAYHNGEJLPKEX-UHFFFAOYSA-N; MolPort-000-653-421; HMS2331F23; STK731712; AKOS001625155; MCULE-9743171840; NCGC00018998-01; NCGC00018998-02; ST067007; SMR000034190; Benzamide, 3,4,5-trimethoxy-N-2-thiazolyl-; Thiazole, 2-(3,4,5-trimethoxybenzoylamino)-; 3,4,5-trimethoxy-N-1,3-thiazol-2-ylbenzamide; SR-01000000944; SR-01000000944-2; Z28173733
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
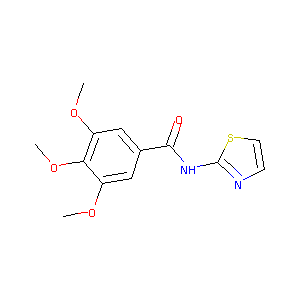 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 294.33 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
