| Drug Name |
ONO-1101
|
| Synonyms |
Landiolol; Landiolol [INN]; ONO-1101; Ono 1101; (S-(R*,R*))-(2,2-Dimethyl-1,3-dioxolan-4-yl)methyl 4-(2-hydroxy-3-((2-((4-morpholinylcarbonyl)amino)ethyl)amino)propoxy)benzenepropanoate; 133242-30-5; 62NWQ924LH; Benzenepropanoic acid, 4-((2S)-2-hydroxy-3-((2-((4-morpholinylcarbonyl)amino)ethyl)amino)propoxy)-, ((4S)-2,2-dimethyl-1,3-dioxolan-4-yl)methyl ester; UNII-62NWQ924LH; [(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl 3-[4-[(2S)-2-hydroxy-3-[2-(morpholine-4-carbonylamino)ethylamino]propoxy]phenyl]propanoate
|
| ATC Code |
- C07AB14: ONO-1101
- C07AB: Beta blocking agents, selective
- C07A: BETA BLOCKING AGENTS
- C07: BETA BLOCKING AGENTS
- C: CARDIOVASCULAR SYSTEM
|
| Structure |
|
 |
|
3D MOL is unavailable
|
2D MOL
|
| #Ro5 Violations (Lipinski): 2 |
Molecular Weight (mw) |
509.6 |
|
| Logarithm of the Partition Coefficient (xlogp) |
0.1 |
| Rotatable Bond Count (rotbonds) |
14 |
| Hydrogen Bond Donor Count (hbonddonor) |
3 |
| Hydrogen Bond Acceptor Count (hbondacc) |
9 |
| Chemical Identifiers |
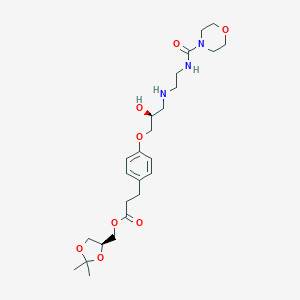
- Formula
- C25H39N3O8
- IUPAC Name
[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]methyl 3-[4-[(2S)-2-hydroxy-3-[2-(morpholine-4-carbonylamino)ethylamino]propoxy]phenyl]propanoate - Canonical SMILES
-
CC1(OCC(O1)COC(=O)CCC2=CC=C(C=C2)OCC(CNCCNC(=O)N3CCOCC3)O)C
- InChI
-
WMDSZGFJQKSLLH-RBBKRZOGSA-N
- InChIKey
-
1S/C25H39N3O8/c1-25(2)35-18-22(36-25)17-34-23(30)8-5-19-3-6-21(7-4-19)33-16-20(29)15-26-9-10-27-24(31)28-11-13-32-14-12-28/h3-4,6-7,20,22,26,29H,5,8-18H2,1-2H3,(H,27,31)/t20-,22+/m0/s1
|
| Cross-matching ID |
- PubChem CID
- 114905
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- INTEDE ID
- DR0914
|
|
|
|
|
|
|
|

