Details of the Drug
General Information of Drug (ID: DMUMHXT)
| Drug Name |
CYC116
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
693228-63-6; CYC-116; CYC116; CHEMBL482967; Kinome_636; 4-methyl-5-(2-(4-morpholinophenylamino)pyrimidin-4-yl)thiazol-2-amine; 4-Methyl-5-(2-((4-morpholinophenyl)amino)-pyrimidin-4-yl)thiazol-2-amine; 4-methyl-5-[2-(4-morpholin-4-ylanilino)pyrimidin-4-yl]-1,3-thiazol-2-amine; 4-methyl-5-(2-((4-morpholinophenyl)amino)pyrimidin-4-yl)thiazol-2-amine; 2uue; CYC 116; GPSZYOIFQZPWEJ-UHFFFAOYSA-N; 2c5t; AC1O4WKE; MLS006011244; SCHEMBL2074998; SYN1034; EX-A753; CYC-116/CYC116; MolPort-009-679-476; HMS3244O12; HMS3244P11; BCPP000273
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
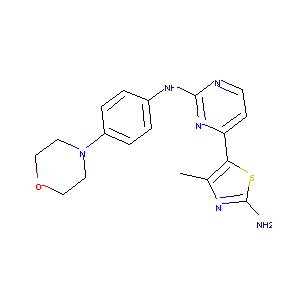 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 368.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
