Details of the Drug
General Information of Drug (ID: DMUQE2A)
| Drug Name |
Dexmethylphenidate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dexmethylphenidate; Dexmethylphenidate (INN); Dexmethylphenidate [INN]; Focalin; Focalin XR; M32RH9MFGP; Methyl D-phenidate; d-Methylphenidate; Attenade; Dex methylphenidate; d-threo-Methylphenidate; dex-methylphenidate; dexmethylphenidatum; dexmetilfenidato; threo-(+)-Methylphenidate; (+)-threo-Methylphenidate; 40431-64-9; CHEBI:51860; CHEMBL827; D-MPH; D-TMP; UNII-M32RH9MFGP; methyl (2R)-2-phenyl-2-[(2R)-piperidin-2-yl]acetate; methyl (2R)-phenyl[(2R)-piperidin-2-yl]acetate; methyl (R)-phenyl[(R)-piperidin-2-yl]acetate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Structure |
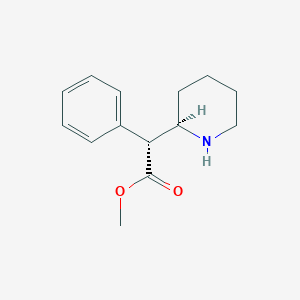 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 233.31 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
