Details of the Drug
General Information of Drug (ID: DMUT9IQ)
| Drug Name |
Bupranolol
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BUPRANOLOL; Betadrenol; Bupranol; Bupranolol [INN:DCF]; Bupranololum; Bupranololum [INN-Latin]; HQIRNZOQPUAHHV-UHFFFAOYSA-N; KL 255; KL-255 [AS HYDROCHLORIDE]; Ophtorenin; SK&F 16805-A; 1-(tert-butylamino)-3-(2-chloro-5-methylphenoxy)propan-2-ol; 14556-46-8; 2-Propanol, 1-(2-chloro-5-methylphenoxy)-3-((1,1-dimethylethyl)amino)-; 2-Propanol, 1-(tert-butylamino)-3-(6-chloro-m-tolyloxy)-; 3-(tert-Butylamino)-1-(6-chloro-m-tolyloxy)-2-propanol; B 1312; BRN 2272923; C14H22ClNO2
|
|||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||
| Structure |
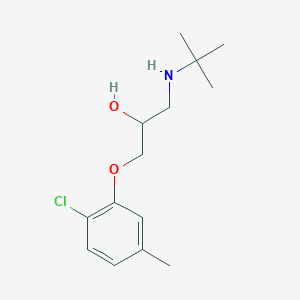 |
|||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 271.78 | ||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.8 | |||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | |||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | |||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
