Details of the Drug
General Information of Drug (ID: DMUWBIJ)
| Drug Name |
Propiverine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
propiverine hydrochloride; 54556-98-8; Propiverine HCl; Mictonorm; Propiverine Hydrochlorride; UNII-DC4GZD10H3; (1-methylpiperidin-4-yl) 2,2-diphenyl-2-propoxyacetate hydrochloride; DSSTox_RID_81957; DSSTox_CID_26847; DSSTox_GSID_46847; C23H29NO3.HCl; DC4GZD10H3; CAS-54556-98-8; NCGC00181103-01; Mictonetten; Pollarine; Mictonorm (TN); propiverin hydrochloride; Bup-4 (TN); AC1L9B4A; CHEBI:8494; SCHEMBL1034248; DTXSID1046847; CHEMBL2359059; Propiverine hydrochloride (JP17); KFUJMHHNLGCTIJ-UHFFFAOYSA-N; BCP09576; Tox21_112719; NSC172140
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
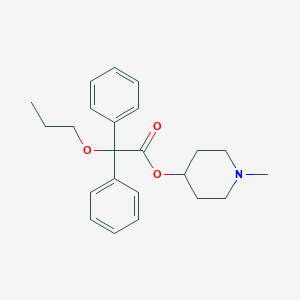 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 367.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
