Details of the Drug
General Information of Drug (ID: DMVTKJZ)
| Drug Name |
BMS-820836
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Liafensine; BMS-820836; UNII-R34ID086Z6; 1198790-53-2; R34ID086Z6; Liafensine [USAN:INN]; Liafensine (USAN); BMS 820836; SCHEMBL1120067; CHEMBL2364614; DTXSID60152610; VCIBGDSRPUOBOG-QFIPXVFZSA-N; AKOS032946316; D10443; 6-[(4s)-2-methyl-4-(2-naphthyl)-1,2,3,4-tetrahydroisoquinolin-7-yl]pyridazin-3-amine; (S)-6-(2-methyl-4-(naphthalen-2-yl)-1,2,3,4-tetrahydroisoquinolin-7-yl)pyridazin-3-amine; (5)-6-(2-methyl-4-(naphthalen-2-yl)-1,2,3,4-tetrahydroisoquinolin-7-yl)pyridazin-3-amine; 3-Pyridazinamine, 6-((4S)-1,2,3,4-tetrahydr
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
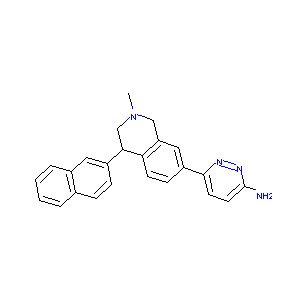 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
