Details of the Drug
General Information of Drug (ID: DMVXBSW)
| Drug Name |
BTZ-043
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BTZ043; 1161233-85-7; BTZ-043; BTZ-10526043; UNII-G55ZH52P57; 2-[(3S)-3-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl]-8-nitro-6-(trifluoromethyl)-1,3-benzothiazin-4-one; Bzt043; MMV676603; SCHEMBL2488829; (S)-2-(2-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl)-8-nitro-6-(trifluoromethyl)-4H-benzo[e][1,3]thiazin-4-one; 4H-1,3-Benzothiazin-4-one, 2-[(2S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]dec-8-yl]-8-nitro-6-(trifluoromethyl)-; G55ZH52P57; 2-[(2S)-2-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl]-8-nitro-6-(trifluoromethyl)-4H-1,3-benzothiazin-4-one; BTZ 043; GTUIRORNXIOHQR-VIFPVBQESA-N; 4H-1,3-BENZOTHIAZIN-4-ONE, 2-((2S)-2-METHYL-1,4-DIOXA-8-AZASPIRO(4.5)DEC-8-YL)-8-NITRO-6-(TRIFLUOROMETHYL)-; BTZ043-racemate; S1097_Selleck; PBTZ 169; CHEMBL1822872; GTPL13034; DTXSID80151286; BCPP000312; EX-A1012; MFCD17215196; AKOS030526000; BCP9000457; CS-5635; AC-35368; AS-74893; BTZ 10526043; HY-13579; EN300-23853592; Q27278777; [2-[(3S)-3-methyl-1,4-dioxa-8-azaspiro[4.5]decan-8-yl]-4-oxo-6-(trifluoromethyl)-1,3-benzothiazin-8-yl]azinic acid; 2-((2S)-2-METHYL-1,4-DIOXA-8-AZASPIRO(4.5)DECAN-8-YL)-8-NITRO-6-TRIFLUOROMETHYL-4H-1,3-BENZOTHIAZIN-4-ONE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
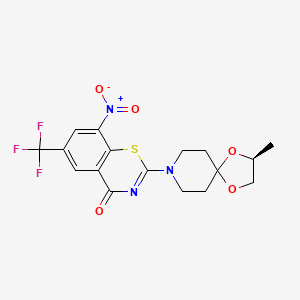 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
