Details of the Drug
General Information of Drug (ID: DMVYNJZ)
| Drug Name |
BUTAPROST
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Butaprost; (R)-Butaprost; 69648-38-0; UNII-HP16WVP23Y; TR-4979; HP16WVP23Y; NCGC00165753-01; Butaprostum; (1R,2R,3R)-3-Hydroxy-2-[(1E,4R)-4-hydroxy-4-(1-propylcyclobutyl)-1-butenyl]-5-oxo-cyclopentaneheptanoic acid methyl ester; Butaprostum [Latin]; Bay q 4218; Butaprost [USAN:INN:BAN]; Butaprost (free acid); AC1NSJVB; Butaprost (USAN/INN); DSSTox_RID_83186; DSSTox_CID_28919; SCHEMBL94764; DSSTox_GSID_48993; CHEMBL271896; GTPL3379; DTXSID8048993; BDBM85602; BAY-Q-4218; ZINC4215145; Tox21_113480; MFCD00867055; 1090AH; PDSP2_001674
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
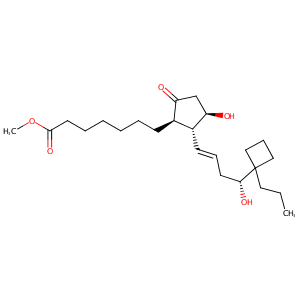 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 408.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 14 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
