Details of the Drug
General Information of Drug (ID: DMW51AX)
| Drug Name |
Anisodamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
anisodamine; racanisodamine; 55869-99-3; (6S)-6-Hydroxyhyoscyamine; (-)-Anisodamine; AC1O6SQS; AC1L4C7W; SCHEMBL620576; ZINC2112569; 8053AH; API0000388; 869A993; Q-100648; [(1S,3S,5S,6S)-6-hydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] (2S)-3-hydroxy-2-phenylpropanoate; (S)-((1S,3S,5S,6S)-6-hydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 3-hydroxy-2-phenylpropanoate; [(1R,3S,5R,6R)-6-hydroxy-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] (2R)-3-hydroxy-2-phenylpropanoate
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
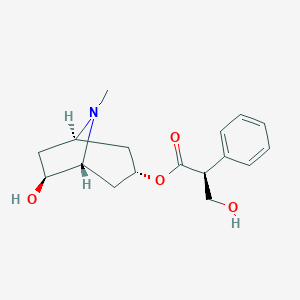 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 305.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
