Details of the Drug
General Information of Drug (ID: DMW73W6)
| Drug Name |
Andrographolide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Andrographolide; 5508-58-7; Andrographis; UNII-410105JHGR; CHEBI:65408; 410105JHGR; (S,E)-4-Hydroxy-3-(2-((1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2(3H)-one; AK-47364; 3-(2-(Decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenenaphthyl)ethylidene)dihydro-4-hydroxyfuran-2(3H)-one; EINECS 226-852-5; NSC 383468; 3alpha,14,15,18-tetrahydroxy-5b,9bH,10a-labda-8(20),12-dien-16-oic acid gamma-Lactone
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
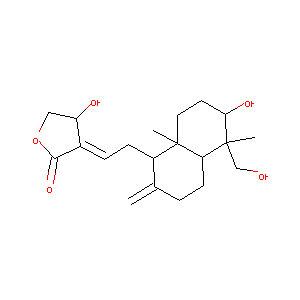 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
