Details of the Drug
General Information of Drug (ID: DMW9R1C)
| Drug Name |
AZD5305
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
AZD5305; Saruparib; 2589531-76-8; AZD-5305; 16MZ1V3RBT; 5-(4-((7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl)piperazin-1-yl)-N-methylpicolinamide; saruparib [INN]; 2-Pyridinecarboxamide, 5-(4-((7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3-yl)methyl)-1-piperazinyl)-N-methyl-; 2-Pyridinecarboxamide, 5-[4-[(7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3-yl)methyl]-1-piperazinyl]-N-methyl-; UNII-16MZ1V3RBT; Azd 5305; CHEMBL5095220; SCHEMBL22912701; GTPL11526; AZD 5305 [WHO-DD]; AZD-5305 [WHO-DD]; EX-A5234; NSC834196; example 4 [WO2021013735]; NSC-834196; AC-37130; MS-26971; SY295016; HY-132167; CS-0163534; E80364; 5-[4-[(7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl]-1-piperazinyl]-N-methylpicolinamide; 5-[4-[(7-ethyl-6-oxo-5H-1,5-naphthyridin-3-yl)methyl]piperazin-1-yl]-N-methylpyridine-2-carboxamide; 5-{4-[(7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3- yl)methyl]piperazin-1-yl}-N-methylpyridine-2- carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
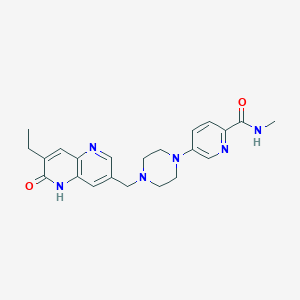 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Prostate cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C82.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
