Details of the Drug
General Information of Drug (ID: DMWAR1T)
| Drug Name |
Ro 61-8048
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
199666-03-0; Ro61-8048; 3,4-dimethoxy-N-(4-(3-nitrophenyl)thiazol-2-yl)benzenesulfonamide; Ro-61-8048; 3,4-dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzenesulfonamide; CHEMBL134915; 3,4-Dimethoxy-N-[4-(3-nitro-phenyl)-thiazol-2-yl]-benzenesulfonamide; CHEBI:34953; C14126; MFCD11040807; 3,4-Dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl]benzenesulfonamide; C17H15N3O6S2; Benzenesulfonamide, 3,4-dimethoxy-N-[4-(3-nitrophenyl)-2-thiazolyl]-; AC1NQZWG; 7ZR; SCHEMBL424256; AOB1234; DTXSID20415218; EX-A805; SYN5225; HMS3886F06; BCP07470; ZINC1546077; 4013AH; BDBM50061916; s8172; AKOS024457509; CCG-222062; CS-3332; NE62855; SB19626; NCGC00370730-07; NCGC00370730-09; NCGC00370730-13; AC-32906; AK312615; AS-16581; DA-43342; HY-12347; B7334; FT-0700256; EC-000.2437; Ro 61-8048, >=98% (HPLC); J-012900; Q27116332; 3,4-Dimethoxy-N-[4-(3-nitrophenyl)-2-thiazolyl]-benzenesulfonamide; 3,4-Dimethoxy-N-[4-(3-nitrophenyl)-2-thiazolyl]benzenesulfonamide; 3,4-dimethoxy-N-[4-(3-nitrophenyl)-1,3-thiazol-2-yl]benzene-1-sulfonamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
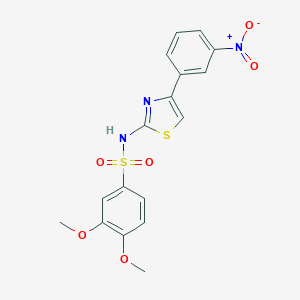 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 421.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
