Details of the Drug
General Information of Drug (ID: DMWPMN2)
| Drug Name |
SAR 444727
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
atuzabrutinib; Atuzabrutinib [INN]; YZ68ZB8LWA; UNII-YZ68ZB8LWA; PRN473; CHEMBL4114766; PRN-473; SAR444727; SAR-444727; 1581714-49-9; (alphaE,3R)-3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1hpyrazolo(3,4-d)pyrimidin-1-yl)-alpha-(2,2-dimethylpropylidene)-beta-oxo-1-piperidinepropanenitrile; 1-Piperidinepropanenitrile, 3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidin-1-yl)-alpha-(2,2-dimethylpropylidene)-beta-oxo-, (alphaE,3R)-; 1-Piperidinepropanenitrile, 3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-alpha-(2,2-dimethylpropylidene)-beta-oxo-, (alphaE,3R)-; ATUZABRUTINIB [USAN]; SCHEMBL15515897; SCHEMBL15516108; GTPL11666; BDBM197260; BDBM50589191; AKOS040756908; compound 11 [PMID: 35302767]; HY-132808; CS-0204055; US9090621, 125A; (2E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile; (alphaE,3R)-3-[4-Amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-alpha-(2,2-dimethylpropylidene)-beta-oxo-1-piperidinepropanenitrile; (E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
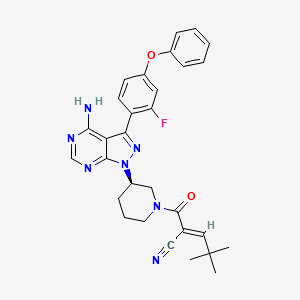 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Atopic dermatitis | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | EA80 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
