Details of the Drug
General Information of Drug (ID: DMX6RZG)
| Drug Name |
EFT508
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Tomivosertib; eFT508; 1849590-01-7; EFT-508; UNII-U2H19X4WBV; U2H19X4WBV; Tomivosertib [INN]; Tomivosertib [USAN]; SCHEMBL17362622; GTPL10167; eFT-508 (eFT508); EFT 508; MolPort-044-560-418; BCP18993; EX-A2494; ZINC575623807; AKOS030627405; CS-5841; compound 23 [PMID: 29526098]; HY-100022; S8275; 6'-((6-aminopyrimidin-4-yl)amino)-8'-methyl-1'H-spiro[cyclohexane-1,3'-imidazo[1,5-a]pyridine]-1',5'(2'H)-dione; 6-[(6-aminopyrimidin-4-yl)amino]-8-methylspiro[2H-imidazo[1,5-a]pyridine-3,1'-cyclohexane]-1,5-dione
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Structure |
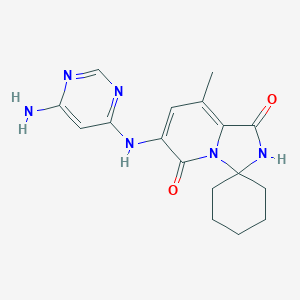 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 340.4 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.3 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
