Details of the Drug
General Information of Drug (ID: DMX9YLR)
| Drug Name |
Pridopidine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
PRIDOPIDINE; 346688-38-8; 4-[3-(Methylsulfonyl)phenyl]-1-propylpiperidine; 4-(3-(Methylsulfonyl)phenyl)-1-propylpiperidine; ACR-16; UNII-HD4TW8S2VK; ACR16; ACR 16; HD4TW8S2VK; CHEMBL596802; FR310826; Pridopidine [USAN:INN]; Pridopidine (USAN/INN); 4-(3-Methanesulfonyl-phenyl)-1-propyl-piperidine; SCHEMBL166748; CTK4H2809; DTXSID90188225; ASP 2314; ZINC22063703; BDBM50308028; AKOS015891431; DB11947; NCGC00386586-01; HY-10684; AS-50146; AJ-80925; FR 310826; DB-014417; AX8258340; CS-0002733; FT-0672149; Huntexil; Pridopidine hydrochloride; ASP-2314; Dopamine modulator (Huntingtons disease), Carlsson Research; Dopamine modulator (Huntingtons disease), NeuroSearch; Dopamine modulator (schizophrenia), Astellas; Dopamine modulator (schizophrenia), Fujisawa; Dopamine modulator (neurological disorders), Merck & Co
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
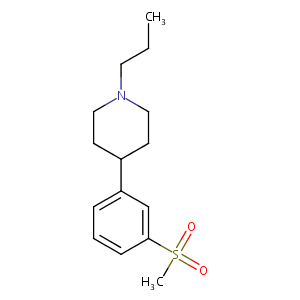 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 281.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Huntington disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A01.10 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
