Details of the Drug
General Information of Drug (ID: DMXC1K9)
| Drug Name |
Alpha 1-PI
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ifenprodil; 23210-56-2; Vadilex; ifenprodil tartrate; Dilvax; Creocral; 4-(2-(4-benzylpiperidin-1-yl)-1-hydroxypropyl)phenol; Ifenprodil [INN:DCF]; RC 61-91; Ifenprodilum [INN-Latin]; UNII-R8OE3P6O5S; EINECS 245-491-4; R8OE3P6O5S; CHEMBL305187; 4-Benzyl-alpha-(p-hydroxyphenyl)-beta-methyl-1-piperidineethanol; 2-(4-Benzylpiperidino)-1-(4-hydroxyphenyl)propanol; 4-[2-(4-benzylpiperidin-1-yl)-1-hydroxypropyl]phenol; NCGC00024643-03; IFENPRODIL HEMITARTRATE; alpha-(4-Hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
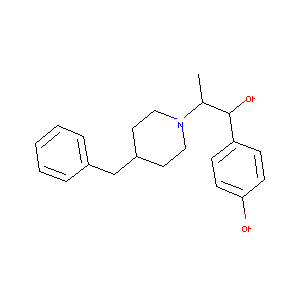 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 325.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
