Details of the Drug
General Information of Drug (ID: DMXCSVH)
| Drug Name |
Oleic acid anilide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Oleanilide; Oleic acid anilide; Oleoylanilide; Oleylanilide; cis-9-Octadecenanilide; Oleyl anilide; (Z)-N-Phenyl-9-octadeceneamide; 5429-85-6; N-Phenyl-9Z-octadecenamide; (Z)-N-phenyl-9-octadecenamide; CHEBI:53721; EINECS 226-584-9; NSC 14233; 9-Octadecenamide, N-phenyl-, (Z)-; BRN 3533033; CHEMBL15611; (9Z)-N-phenyloctadec-9-enamide; Oleic anilide; C24H39NO; Oleanilide (7CI); AC1NS5WI; Epitope ID:117134; 2-12-00-00150 (Beilstein Handbook Reference); SCHEMBL4143902; (Z)-N-phenyloctadec-9-enamide; Octadec-9-enoic acid phenylamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
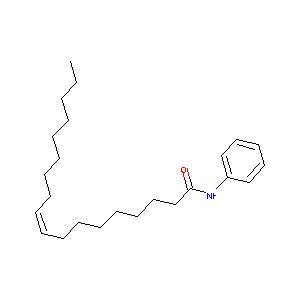 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 357.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 8.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 16 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
