Details of the Drug
General Information of Drug (ID: DMXD93K)
| Drug Name |
ARRY-614
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Pexmetinib; 945614-12-0; ARRY-614; Pexmetinib (ARRY-614); UNII-3750D0U8B5; 3750D0U8B5; Pexmetinib [INN]; Pexmetinib;ARRY-614; Pexmetinib(ARRY-614); ARRY614; SCHEMBL379035; GTPL9917; MolPort-039-193-822; LNMRSSIMGCDUTP-UHFFFAOYSA-N; BCP28410; EX-A1421; s7799; ZINC41747181; AKOS032945154; SB16914; Urea, N-(3-(1,1-dimethylethyl)-1-(4-methylphenyl)-1H-pyrazol-5-yl)-N'-((5-fluoro-2-((1-(2-hydroxyethyl)-1H-indazol-5-yl)oxy)phenyl)methyl)-
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
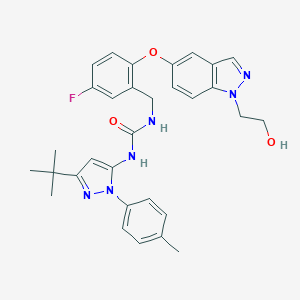 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 556.6 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
