Details of the Drug
General Information of Drug (ID: DMXI8CD)
| Drug Name |
TRC-253
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | OUEHJEYKNYQVRC-UHFFFAOYSA-N; TRC253; SCHEMBL19128768; EX-A1808; 2110426-27-0; 5-(8-oxo-5-(6-(piperidin-4-yloxy)pyridin-3-yl)-6-thioxo-5,7-diazaspiro[3.4]octan-7-yl)-3-(trifluoromethyl)picolinonitrile | ||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Structure |
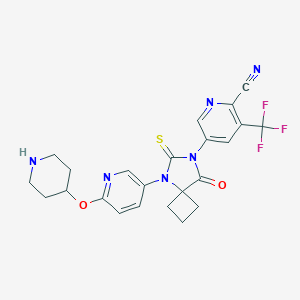 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 502.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 10 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| ICD Disease Classification | 02 Neoplasm | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disease Class | ICD-11: 2C82 Prostate cancer | |||||||||||||||||||||||
| The Studied Tissue | Prostate | |||||||||||||||||||||||
| The Studied Disease | Prostate cancer [ICD-11:2C82] | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
