Details of the Drug
General Information of Drug (ID: DMXZKV7)
| Drug Name |
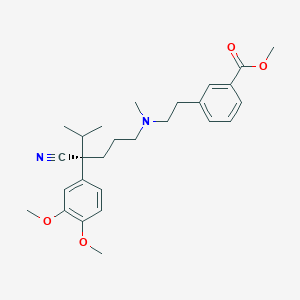
Etripamil
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
UNII-S82A18Y42P; S82A18Y42P; 1593673-23-4; Etripamil [USAN:INN]; Etripamil (USAN/INN); CHEMBL3707312; DB12605; Benzoic acid, 3-(2-(((4S)-4-cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl)methylamino)ethyl)- , methyl ester; D10932
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Structure |
 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
References
