| Synonyms |
UNII-OK43YC00I7; OK43YC00I7; LY53857; LY 53857; LY-53857 free base; Biomol-NT_000165; AC1MHY97; Lopac0_000721; GTPL183; BPBio1_000595; CHEMBL1356280; CCG-204806; NCGC00163176-01; LY-53,857; LS-187317; Ergoline-8beta-carboxylic acid, 1-isopropyl-6-methyl-, 2-hydroxy-1-methylpropyl ester; 6-Methyl-1-isopropylergoline-8beta-carboxylic acid 2-hydroxy-1-methylpropyl ester; 6-methyl-1-(1-methylethyl)ergoline-8beta-carboxylic acid 2-hydroxy-1-methylpropyl ester; Ergoline-8-carboxylic acid, 6-methyl-1-(1-methylethyl)-, 2-hydroxy-1-m
|
| Chemical Identifiers |
- Formula
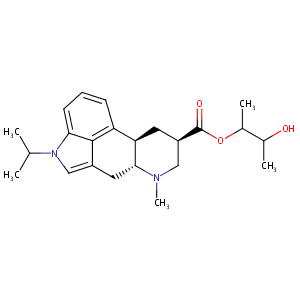
- C23H32N2O3
- IUPAC Name
3-hydroxybutan-2-yl (6aR,9R,10aR)-7-methyl-4-propan-2-yl-6,6a,8,9,10,10a-hexahydroindolo[4,3-fg]quinoline-9-carboxylate - Canonical SMILES
-
CC(C)N1C=C2C[C@@H]3[C@H](C[C@H](CN3C)C(=O)OC(C)C(C)O)C4=C2C1=CC=C4
- InChI
-
InChI=1S/C23H32N2O3/c1-13(2)25-12-16-10-21-19(18-7-6-8-20(25)22(16)18)9-17(11-24(21)5)23(27)28-15(4)14(3)26/h6-8,12-15,17,19,21,26H,9-11H2,1-5H3/t14?,15?,17-,19-,21-/m1/s1
- InChIKey
-
JQYLIGHHVGCTPR-LYRPIDSHSA-N
|