Details of the Drug
General Information of Drug (ID: DMYBIFS)
| Drug Name |
Parsaclisib
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
INCB050465; Parsaclisib free base; UNII-OS7097575K; 1426698-88-5 (free base); OS7097575K; 1426698-88-5; (4R)-4-{3-[(1S)-1-(4-amino-3-methyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)ethyl]-5-chloro-2-ethoxy-6-fluorophenyl}pyrrolidin-2-one; INCB-050465; (4R)-4-(3-((1S)-1-(4-Amino-3-methyl-1H-pyrazolo(3,4-d)pyrimidin-1-yl)ethyl)-5-chloro-2-ethoxy-6-fluorophenyl)pyrrolidin-2-one; Parsaclisib [INN]; Parsaclisib [USAN]; Parsaclisib (USAN/INN); Parsaclisib [USAN:INN]; INCB050465 free base; INCB-050465 free base; CHEMBL4297615; SCHEMBL14736228; BDBM272573; EX-A2638; WHO 10589; DB14867; US10065963, 32c; HY-109068; CS-0033435; D11437; (R)-4-(3-((S)-1-(4-amino-3-methyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl)ethyl)-5-chloro-2-ethoxy-6-fluorophenyl)pyrrolidin-2-one; 2-Pyrrolidinone, 4-(3-((1S)-1-(4-amino-3-methyl-1H-pyrazolo(3,4-d)pyrimidin-1-yl)ethyl)-5-chloro-2-ethoxy-6-fluorophenyl)-, (4R)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
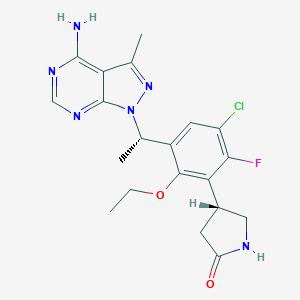 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 432.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Follicular lymphoma | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A80 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
