Details of the Drug
General Information of Drug (ID: DMYLFQR)
| Drug Name |
BMS-986120
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1478712-37-6; UNII-WDT28B7071; WDT28B7071; 4-(4-(((6-methoxy-2-(2-methoxyimidazo[2,1-b][1,3,4]thiadiazol-6-yl)benzofuran-4-yl)oxy)methyl)-5-methylthiazol-2-yl)morpholine; 4-[4-[[6-methoxy-2-(2-methoxyimidazo[2,1-b][1,3,4]thiadiazol-6-yl)-1-benzofuran-4-yl]oxymethyl]-5-methyl-1,3-thiazol-2-yl]morpholine; 4-(4-(((6-Methoxy-2-(2-methoxyimidazo(2,1-b)(1,3,4)thiadiazol-6-yl)benzofuran-4-yl)oxy)methyl)-5-methylthiazol-2-yl)morpholine; CHEMBL3716726; SCHEMBL15348871; GTPL10269; BDBM176061; EX-A3977; BMS986120; HY-19837; CS-0016978; US9688695, 94; 2-methoxy-6-[6-methoxy-4-[[5-methyl-2-(4-morpholinyl)-4-thiazolyl]methoxy]-2-benzofuranyl]-imidazo[2,1-b]-1,3,4-thiadiazole; Imidazo(2,1-b)-1,3,4-thiadiazole, 2-methoxy-6-(6-methoxy-4-((5-methyl-2-(4-morpholinyl)-4-thiazolyl)methoxy)-2-benzofuranyl)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
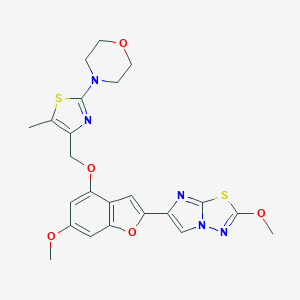 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 513.6 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
