Details of the Drug
General Information of Drug (ID: DMYPLZ8)
| Drug Name |
Cristacarpin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cristacarpin; Erythrabyssin I; 74515-47-2; Crystacarpin; CHEMBL454849; CHEBI:3917; AC1L2QIR; AC1Q70TT; SCHEMBL4740053; MolPort-005-945-627; ZINC4098607; LMPK12070114; BDBM50317430; 9166AF; 6h-benzofuro(3,2-c)(1)benzopyran-3,6a(11ah)-diol, 9-methoxy-10-(3-methyl-2-butenyl)-,(6as-cis)-; C10206; 6H-Benzofuro(3,2-c)(1)benzopyran-3,6a(11aH)-diol, 9-methoxy-10-(3-methyl-2-butenyl)-, (6aS-cis)-; 6H-Benzofuro[3,2-c][1]benzopyran-3,6a(11aH)-diol,9-methoxy-10-(3-methyl-2-buten-1-yl)-, (6aS,11aS)-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
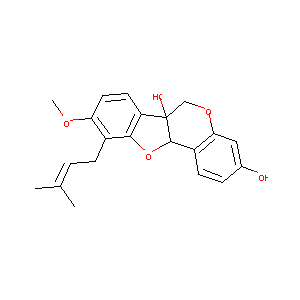 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 354.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
