Details of the Drug
General Information of Drug (ID: DMYTGW0)
| Drug Name |
Celiprolol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Celectol; Celiprolol [INN:BAN]; Celiprololum; Celiprololum [INN-Latin]; RHC 5320 A; RHC-5320A; ST-1396; Selectol; UL/1677; celiprolol; 3-[3-acetyl-4-[3-(tert-butylamino)-2-hydroxypropoxy]phenyl]-1,1-diethylurea; 56980-93-9; BRN 2776298; CCRIS 3400; EINECS 260-497-7; N'-(3-Acetyl-4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)phenyl)-N,N-diethylurea; Urea, N'-(3-acetyl-4-(3-((1,1-dimethylethyl)amino)-2-hydroxypropoxy)phenyl)-N,N-diethyl-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Structure |
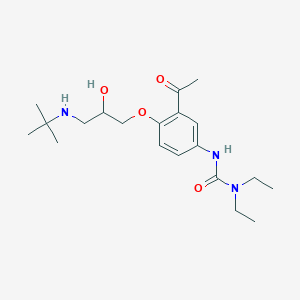 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 379.5 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
