Details of the Drug
General Information of Drug (ID: DMZ43N1)
| Drug Name |
N-adamantyl-N'-cyclohexylurea
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CHEMBL242255; N-1-adamantyl-N'-cyclohexylurea; AC1NMPQQ; Urea-based compound, 13; ChemDiv3_000178; Oprea1_419513; 1-adamantyl-3-cyclohexylurea; MLS000719875; SCHEMBL653168; BDBM25732; MolPort-003-713-177; HMS2616F12; HMS1473I02; 3-adamantan-1-yl-1-cyclohexylurea; 1-(1-adamantyl)-3-cyclohexylurea; ZINC3895072; AKOS001483388; MCULE-6291906995; 1-(Adamantane-1-yl)-3-cyclohexylurea; IDI1_019496; N-adamantanyl(cyclohexylamino)carboxamide; NCGC00177652-01; SMR000304404; EU-0000129; ST51030996; US8815951, 192; SR-01000389488
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
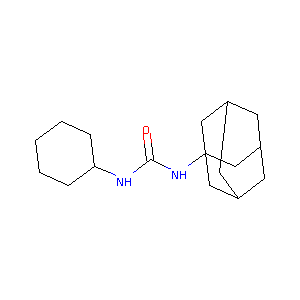 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 276.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
