Details of the Drug
General Information of Drug (ID: DMZARP1)
| Drug Name |
FV-100
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CF-1001; CF-1094; CF-1368; CF-1369; CF-1449; CF-1452; CF-1698; CF-1712; CF-1743; CF-1821; CF-1835; CF-1837; CF-1838; CF-1851; CF-2004; CF-2160; CF-2161; CF-2200; BCNAs (antiviral), FermaVir Pharmaceuticals; Bicyclic nucleoside analogs (VZV infection), FermaVir; Bicyclic nucleoside analogs (VZV infection), Inhibitex; Antivirals (nucleoside derivatives), Welsh School of Pharmacy/Rega; BCNAs (antiviral), Rega/Welsh School of Pharmacy; Bicyclic nucleoside analogs (antiviral), Rega/Welsh School of Pharmacy
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
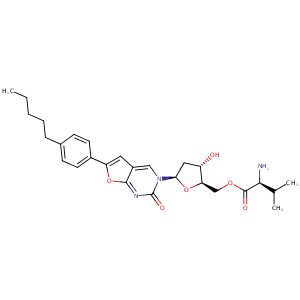 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 |
Molecular Weight | 534 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 11 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Varicella zoster virus infection | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 1E91 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
