Details of the Drug
General Information of Drug (ID: DMZGX27)
| Drug Name |
Mesdopetam
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
mesdopetam; IRL790; IRL-790; HD4QV8GX26; 1403894-72-3; UNII-HD4QV8GX26; N-[2-(3-fluoro-5-methylsulfonylphenoxy)ethyl]propan-1-amine; 1-Propanamine, N-(2-(3-fluoro-5-(methylsulfonyl)phenoxy)ethyl)-; MESDOPETAM [INN]; MESDOPETAM [WHO-DD]; CHEMBL4594447; SCHEMBL13732678; GTPL10811; OSBPYFBXSLJHCR-UHFFFAOYSA-N; AKOS040756735; HY-109150; CS-0115974; N-[2-(3-FLUORO-5-METHYLSULFONYL-PHENOXY)ETHYL]PROPAN-1-AMINE; N-{2-[3-FLUORO-5-(METHYLSULFONYL)PHENOXY]ETHYL}-N-PROPYLAMINE
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||
| Structure |
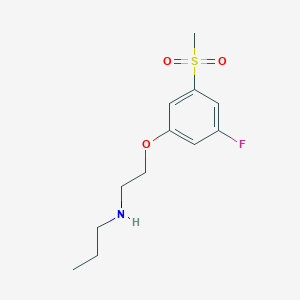 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
