Details of the Drug
General Information of Drug (ID: DMZI3WO)
| Drug Name |
Etomidate
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Absele; Amidate; Ethnor; Ethomidate; Etomidato; Etomidatum; Hypnomidate; Radenarcon; Radenarkon; Etomidic acid; R 16659; R 26490; R26490; Amidate (TN); Amidate (pharmaceutical); Amidate, Etomidate; D-Etomidate; Etomidato [INN-Spanish]; Etomidatum [INN-Latin]; R-26490; Etomidate (USAN/INN); Etomidate [USAN:BAN:INN]; Ethyl 3-(1-phenylethyl)imidazole-4-carboxylate; Ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate; R-(+)-Ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate; (+)-Ethyl 1-(alpha-methylbenzyl)imidazole-5-carboxylate; (+)-Etomidate; (R)-(+)-1-(alpha-Methylbenzyl)imidazole-5-carboxylic acid ethyl ester; (d)-Etomidate; 1-(1-Phenylethyl)-1H-imidazole-5-carboxylic acid ethyl ester; 1-(1-Phenylethyl)-imidazole-5-carboxylic acid, ethyl ester; 1-(alpha-Methylbenzyl)-1H-imidazole-5-carboxylic acid ethyl ester; 1H-Imidazole-5-carboxylic acid, 1-((1R)-1-phenylethyl)-, ethyl ester; 3-(1-Phenyl-ethyl)-3H-imidazole-4-carboxylic acid ethyl ester
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Analgesics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
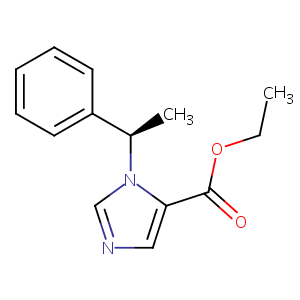 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 244.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Etomidate
Coadministration of a Drug Treating the Disease Different from Etomidate (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5463). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS applied to over 900 drugs | ||||
| 3 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 4 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | ||||
| 6 | Gill SS, Wright EM, Reilly CS "Pharmacokinetic interaction of propofol and fentanyl: single bolus injection study." Br J Anaesth 65 (1990): 760-5. [PMID: 2265045] | ||||
| 7 | Warrington SJ, Ankier SI, Turner P "Evaluation of possible interactions between ethanol and trazodone or amitriptyline." Neuropsychobiology 15 (1986): 31-7. [PMID: 3725002] | ||||
| 8 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
