Details of the Drug
General Information of Drug (ID: DMZK70E)
| Drug Name |
BN 50730
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Rocepafant; BN 50730; UNII-4KGX1STY2N; BN-50730; 4KGX1STY2N; 132579-32-9; BN50730; 132418-36-1; 6-(2-Chlorophenyl)-9-((4-methoxyphenyl)thiocarbamoyl)-1-methyl-7,8,9,10-tetrahydro-4H-pyrido(4',3'-4,5)thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine; 4H-Pyrido(4',3'-4,5)thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine-9(8H)-carbothioamide, 6-(2-chlorophenyl)-7,10-dihydro-N-(4-methoxyphenyl)-1-methyl-; Rocepafant [INN]; LAU8080; LAU-8080; LAU 8080; AC1MHWNG; C27H25ClN6OS; SCHEMBL1065775; CHEMBL2105298; ZINC1481916; LS-172840
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
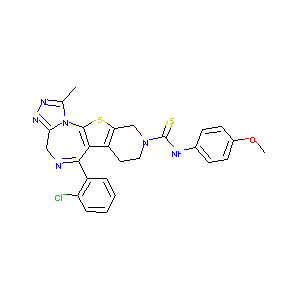 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 535.1 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Asthma | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | CA23 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
