Details of the Drug
General Information of Drug (ID: DMZSJRC)
| Drug Name |
8-acetyl-7-methoxy-2H-chromen-2-one
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
8-Acetyl-7-methoxycoumarin; 8-acetyl-7-methoxy-2H-chromen-2-one; 89019-07-8; CHEMBL1288594; 8-Acetyl-7-methoxy-chromen-2-one; 2H-1-Benzopyran-2-one,8-acetyl-7-methoxy-; acetyl methoxycoumarin; ACMC-20lgfq; acetyl-7-methoxycoumarin, 8-; SCHEMBL16283617; CTK5G2445; DTXSID20556130; 8-acetyl-7-methoxy-2-oxochromene; 8-acetyl-7-methoxycoumarin ,98%; ZINC2562448; MFCD00270164; 8 - Acetyl - 7 - methoxycoumarin; BDBM50332024; 7-methoxy-8-coumarinul methyl ketone; AKOS016009510; RP27113; ST085908; AJ-40748; DB-078289; KB-200257; AX8111029
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
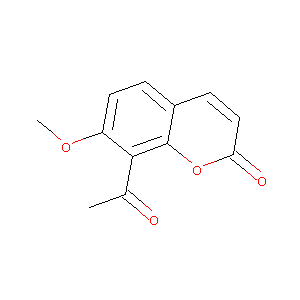 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 218.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
