Details of the Drug
General Information of Drug (ID: DMZTOJ6)
| Drug Name |
TBA-7371
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1494675-86-3; DprE1-IN-1; AZ7371; N-(2-hydroxyethyl)-1-((6-methoxy-5-methylpyrimidin-4-yl)methyl)-6-methyl-1H-pyrrolo[3,2-b]pyridine-3-carboxamide; UNII-R3T98GBE3C; AZ-7371; R3T98GBE3C; CHEMBL3109802; 1H-Pyrrolo(3,2-b)pyridine-3-carboxamide, N-(2-hydroxyethyl)-1-((6-methoxy-5-methyl-4-pyrimidinyl)methyl)-6-methyl-; 1H-Pyrrolo[3,2-b]pyridine-3-carboxamide, N-(2-hydroxyethyl)-1-[(6-methoxy-5-methyl-4-pyrimidinyl)methyl]-6-methyl-; ZA7371; AZ-7371DprE1-IN-1; AZ7371;DprE1-IN-1; SCHEMBL16395186; EX-A777; BCP16818; BDBM50019654; MFCD29477419; ZINC103248024; CS-5414; ZA-7371; HY-19750; FT-0700212; J-690193; N-(2-Hydroxyethyl)-1-((6-methoxy-5-methylpyrimidin-4-yl)methyl)-6- methyl-1H-pyrrolo(3,2-b)pyridine-3-carboxamide; N-(2-hydroxyethyl)-1-[(6-methoxy-5-methyl-pyrimidin-4-yl)methyl]-6-methyl-pyrrolo[3,2-b]pyridine-3-carboxamide; N-(2-hydroxyethyl)-1-[(6-methoxy-5-methylpyrimidin-4-yl)methyl]-6-methylpyrrolo[3,2-b]pyridine-3-carboxamide
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
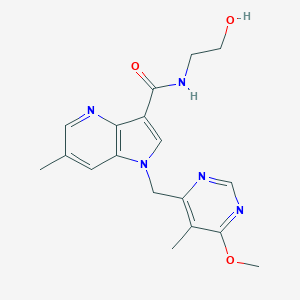 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 355.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
