| 1 |
ClinicalTrials.gov (NCT02941523) Safety, PK and Efficacy of NOX66 as a Monotherapy and Combined With Carboplatin in Refractory Solid Tumours
|
| 2 |
ClinicalTrials.gov (NCT00382811) OVATURE (OVArian TUmor REsponse) A Phase III Study of Weekly Carboplatin With and Without Phenoxodiol in Patients With Platinum-Resistant, Recurrent Epithelial Ovarian Cancer. U.S. National Institutes of Health.
|
| 3 |
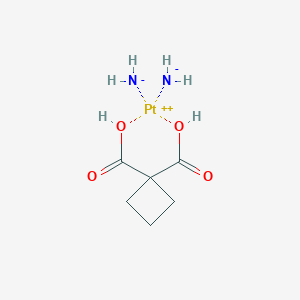
Carboplatin FDA Label
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7624).
|
| 5 |
Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006 Feb 1;106(3):599-608. doi: 10.1002/cncr.21633.
|
| 6 |
Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41.
|
| 7 |
Reciprocal relationship between cytosolic NADH and ENOX2 inhibition triggers sphingolipid-induced apoptosis in HeLa cells. J Cell Biochem. 2010 Aug 15;110(6):1504-11. doi: 10.1002/jcb.22724.
|
| 8 |
Inhibition of carboplatin-induced DNA interstrand cross-link repair by gemcitabine in patients receiving these drugs for platinum-resistant ovarian cancer.Clin Cancer Res.2010 Oct 1;16(19):4899-905.
|
| 9 |
Overcoming platinum drug resistance with copper-lowering agents. Anticancer Res. 2013 Oct;33(10):4157-61.
|
| 10 |
PharmGKB: A worldwide resource for pharmacogenomic information. Wiley Interdiscip Rev Syst Biol Med. 2018 Jul;10(4):e1417. (ID: PA150642262)
|
|
|
|
|
|
|