| 1 |
ClinicalTrials.gov (NCT00209742) Randomized Phase III Adjuvant Study for Stage III Colorectal Cancer
|
| 2 |
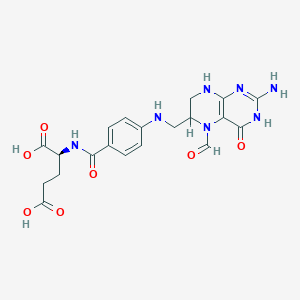
Leucovorin FDA Label
|
| 3 |
Clinical pipeline report, company report or official report of Taiho Pharmaceutical.
|
| 4 |
Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17-(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003 Jul 15;63(14):4048-54.
|
| 5 |
Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: effect of polyglutamylation on MTX transport. Cancer Res. 2001 Oct 1;61(19):7225-32.
|
| 6 |
Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002 Jun 1;62(11):3144-50.
|
| 7 |
UFT Capsules (uracil-tegafur).
|
| 8 |
Schedule-dependent therapeutic effects of gemcitabine combined with uracil-tegafur in a human pancreatic cancer xenograft model. Pancreas. 2006 Aug;33(2):142-7. doi: 10.1097/01.mpa.0000226882.48204.26.
|
|
|
|
|
|
|