| 1 |
ClinicalTrials.gov (NCT03687073) Trial of Indole-3-Carbinol and Silibinin
|
| 2 |
ClinicalTrials.gov (NCT02525159) Effectiveness of DIM Supplements to Increase 2-OHE1/16 Ratio (EDIMI216OHE1). U.S. National Institutes of Health.
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
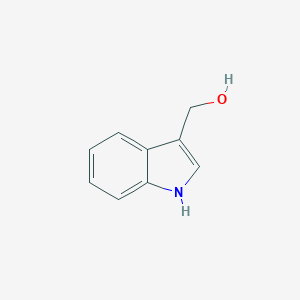
Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther. 2007 Nov;6(11):3071-9. doi: 10.1158/1535-7163.MCT-07-0117.
|
| 5 |
Anticarcinogenic effect of indole-3-carbinol (I3C) on human hepatocellular carcinoma SNU449 cells. Hum Exp Toxicol. 2019 Jan;38(1):136-147. doi: 10.1177/0960327118785235. Epub 2018 Jul 11.
|
| 6 |
Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005 Sep;4(9):1201-15. doi: 10.4161/cc.4.9.1993. Epub 2005 Sep 6.
|
| 7 |
Protective effect of Indole-3-carbinol, an NF-B inhibitor in experimental paradigm of Parkinson's disease: In silico and in vivo studies. Brain Behav Immun. 2020 Nov;90:108-137.
|
| 8 |
Induction of phase-1 metabolizing enzymes by oltipraz, flavone and indole-3-carbinol enhance the formation and transport of benzo[a]pyrene sulfate conjugates in intestinal Caco-2 cells. Toxicol Lett. 2005 Aug 14;158(2):140-51.
|
| 9 |
The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim Pol. 2007;54(1):113-7.
|
| 10 |
Protective effects of isothiocyanates alone or in combination with vitamin C towards N-nitrosodibutylamine or N-nitrosopiperidine-induced oxidative DNA damage in the single-cell gel electrophoresis (SCGE)/HepG2 assay. J Appl Toxicol. 2008 Mar;28(2):196-204.
|
| 11 |
Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells. Arch Toxicol. 2017 May;91(5):2209-2221.
|
| 12 |
In vitro analysis of factors influencing CYP1A2 expression as potential determinants of interindividual variation. Pharmacol Res Perspect. 2017 Mar 2;5(2):e00299.
|
| 13 |
1-Benzyl-indole-3-carbinol is a novel indole-3-carbinol derivative with significantly enhanced potency of anti-proliferative and anti-estrogenic properties in human breast cancer cells. Chem Biol Interact. 2010 Aug 5;186(3):255-66. doi: 10.1016/j.cbi.2010.05.015. Epub 2010 Jun 2.
|
| 14 |
Indole-3-carbinol is a negative regulator of estrogen receptor-alpha signaling in human tumor cells. J Nutr. 2000 Dec;130(12):2927-31. doi: 10.1093/jn/130.12.2927.
|
| 15 |
Combination of xanthohumol and phenethyl isothiocyanate inhibits NF-B and activates Nrf2 in pancreatic cancer cells. Toxicol In Vitro. 2020 Jun;65:104799. doi: 10.1016/j.tiv.2020.104799. Epub 2020 Feb 15.
|
| 16 |
Inhibition of MUC1 expression by indole-3-carbinol. Int J Cancer. 2004 May 10;109(6):810-6. doi: 10.1002/ijc.20031.
|
| 17 |
Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood. 2005 Jul 15;106(2):641-9. doi: 10.1182/blood-2004-12-4589. Epub 2005 Apr 5.
|
| 18 |
N-Alkoxy derivatization of indole-3-carbinol increases the efficacy of the G1 cell cycle arrest and of I3C-specific regulation of cell cycle gene transcription and activity in human breast cancer cells. Biochem Pharmacol. 2008 Feb 1;75(3):713-24. doi: 10.1016/j.bcp.2007.09.024. Epub 2007 Oct 2.
|
| 19 |
BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006 Feb 13;94(3):407-26. doi: 10.1038/sj.bjc.6602935.
|
| 20 |
A Gene Expression Biomarker Predicts Heat Shock Factor 1 Activation in a Gene Expression Compendium. Chem Res Toxicol. 2021 Jul 19;34(7):1721-1737. doi: 10.1021/acs.chemrestox.0c00510. Epub 2021 Jun 25.
|
| 21 |
Agonistic effects of diverse xenobiotics on the constitutive androstane receptor as detected in a recombinant yeast-cell assay. Toxicol In Vitro. 2018 Feb;46:335-349. doi: 10.1016/j.tiv.2017.09.014. Epub 2017 Sep 18.
|
| 22 |
Indole-3-carbinol and 3,3'-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun. 2005 Mar 4;328(1):63-9. doi: 10.1016/j.bbrc.2004.12.138.
|
| 23 |
Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. Toxicol Sci. 2007 Apr;96(2):227-36. doi: 10.1093/toxsci/kfl147. Epub 2006 Oct 31.
|
|
|
|
|
|
|