| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
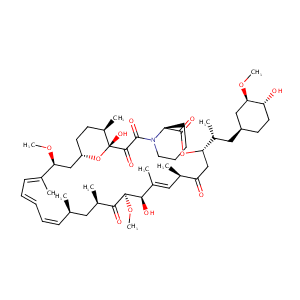
Sirolimus FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 201578.
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 5 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6031).
|
| 6 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 7 |
ClinicalTrials.gov (NCT04341675) Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia. U.S. National Institutes of Health.
|
| 8 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5688).
|
| 9 |
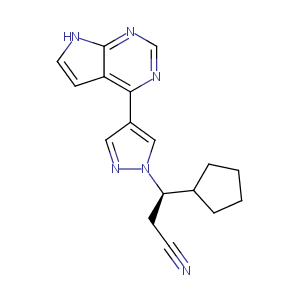
Ruxolitinib FDA Label
|
| 10 |
Incyte begins Phase III trial of ruxolitinib to treat Covid-19. 20.April.2020.
|
| 11 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 12 |
Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51.
|
| 13 |
The genomic landscape of nasopharyngeal carcinoma.Nat Genet. 2014 Aug;46(8):866-71.
|
| 14 |
Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020 Mar 16;6:14.
|
| 15 |
Pharmacogenetics of tacrolimus and sirolimus in renal transplant patients: from retrospective analyses to prospective studies. Transplant Proc. 2007 Sep;39(7):2142-4.
|
| 16 |
Pharmacokinetic and pharmacodynamic interactions between the immunosuppressant sirolimus and the lipid-lowering drug ezetimibe in healthy volunteers. Clin Pharmacol Ther. 2010 Jun;87(6):663-7.
|
| 17 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 18 |
Drug Interactions Flockhart Table
|
| 19 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 20 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 21 |
Urokinase-type plasminogen activator receptor signaling is critical in nasopharyngeal carcinoma cell growth and metastasis.Cell Cycle. 2014;13(12):1958-69.
|
| 22 |
The Use of Anti-Inflammatory Drugs in the Treatment of People With Severe Coronavirus Disease 2019 (COVID-19): The Perspectives of Clinical Immunologists From China. Clin Immunol. 2020 May;214:108393.
|
|
|
|
|
|
|